Using Artificial Intelligence (AI) on ASTM standards and related intellectual property is prohibited. Violations will result in suspension of access.
Jul 21, 2025
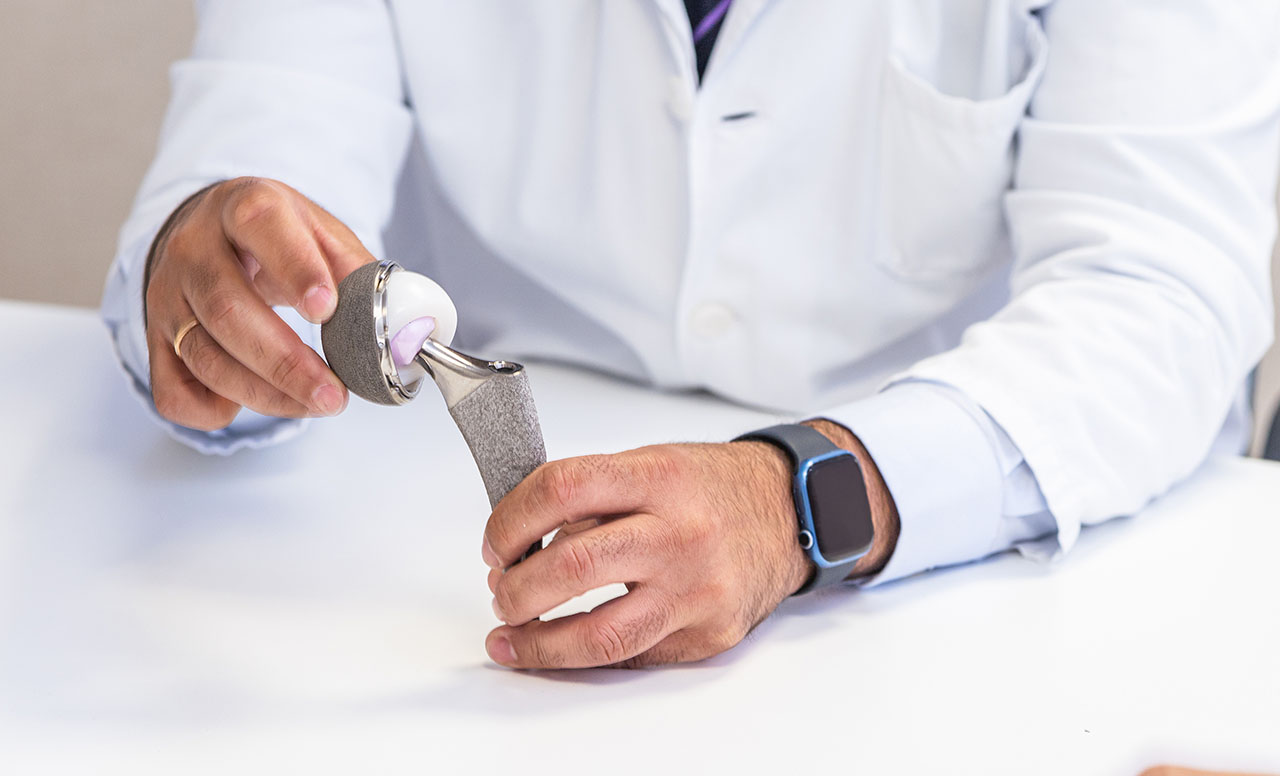
ASTM International has approved a new standard that will help ensure hip joint replacements by simulating harsh conditions in a laboratory setting (F3738).
The new standard was developed by the arthroplasty subcommittee (F04.22), part of ASTM’s medical and surgical materials and devices committee (F04).
According to ASTM members Leah Guenther, technology leader for orthopedics at Lucideon, and Elizabeth Hippensteel, staff scientist, kinematics at J&J Medtech, the new standard enables the direct comparison of metal-on-polyethylene hip replacements under standardized third-body particle wear conditions, which is just one type of worst-case wear scenario.
“In the body, small destructive particles (either from the patient, surgery, or from the device) can get into the joint space,” says Guenther. “These ‘third-body particles’ can potentially affect the function and longevity of the hip replacement, leading to implant failure and additional surgeries. These additional surgeries aren’t just costly—they can be hard on the patient’s body, increase the risk of complications, and lead to longer recovery times, especially in older adults.”
Guenther and Hippensteel add that this test method also supports the development and comparison of better materials and designs that can stand up to everyday use and unexpected challenges.
ASTM welcomes participation in the development of its standards. Become a member at www.astm.org/JOIN.
September / October 2025